3.1 Airways disease, obstructive
| First line drugs | Second line drugs | Specialist drugs | Secondary care drugs |
Traffic light status (TLS) explained:
- Green: Routine prescribing within licensed indication
- Amber 1: specialist recommendation followed by GP initiation and continuation
- Amber 2: specialist or GP initiation in line with local guideline after 1st line failure followed by GP continuation
- Amber 3: specialist initiation and stabilisation followed by GP continuation
- Amber SCG: specialist initiation and stabilisation followed by GP continuation in line with an agreed shared care guideline
- Red: Hospital or specialist prescribing only
Antimuscarinics (inhaled)
Short Acting Muscarinic Antagonist - SAMA
Ipratropium bromide
- Inhaler 20 micrograms/dose
- Nebuliser solution 250 micrograms in 1mL, 500 micrograms in 2mL
Notes:
- Patients must be instructed in the correct administration of ipratropium nebuliser solution.
- Care must be taken not to allow the solution or mist to enter the eyes.
- It is recommended that the nebulised solution be administered via a mouthpiece. If this is not available and a nebuliser mask is used, it must fit properly.
- Patients who may be predisposed to glaucoma should be warned specifically to protect their eyes.
Long Acting Muscarinic Antagonist - LAMA
Braltus® (tiotropium)
- Braltus 10 micrograms delivered dose inhalation powder hard capsule (Zonda Inhaler device).
Notes:
- First line most cost effective choice.
- Each capsule contains 16 micrograms of tiotropium bromide equivalent to 13 micrograms of tiotropium. The delivered dose (the dose that leaves the mouthpiece of the Zonda inhaler) is 10 micrograms of tiotropium per capsule.
- Each patient should be issued a new Zonda inhaler and capsules every 30 days; old inhalers should be discarded
- Braltus Zonda inhaler delivers the same dose of Tiotropium as Spiriva HandiHaler (18 micrograms – delivered dose of 10 micrograms)
Eklira® Genuair (aclidinium bromide)
- Inhalation powder, 375 micrograms (≡ aclidinium 322 micrograms)/inhalation (delivered dose),
Glycopyrronium bromide
- Inhalation powder, hard capsule, (for use with Seebri Breezhaler® device), 50 micrograms.
Spiriva® (tiotropium)
- Respimat (solution for inhalation) 2.5 micrograms/metered inhalation
Notes:
- Tiotropium is included as an option in the management of COPD.
- Spiriva Respimat has been included for use in patients in whom tiotropium is an appropriate choice of maintenance bronchodilator treatment but should be restricted to patients who have poor manual dexterity and therefore have difficulty using the Braltus Zonda device.
- Patients with COPD who use tiotropium should be reminded not to exceed the recommended once-daily dose of:
Two puffs Spiriva Respimat 2.5 micrograms
Incruse Ellipta® (umeclidinium)
- Inhalation powder, 55 micrograms (≡ umeclidinium 55 micrograms)/inhalation (delivered dose)
Short Acting Beta2 Agonist - SABA
Salbutamol
- Inhaler CFC free 100 micrograms/metered inhalation
- Easyhaler 100 micrograms/ metered inhalation
- Dry powder for inhalation, 100 micrograms/metered inhalation
- Nebuliser solution 2.5mg in 2.5mL, 5mg in 2.5mL
- Oral solution (Ventolin) 2mg/5mL
- Tablets 2mg, 4mg
- Modified-release capsules 4mg, 8mg
- Injection 500 micrograms/1mL (Hospital only)
- Solution for IV infusion 5mg in 5mL (Hospital only)
Bricanyl® (terbutaline)
- Turbohaler® 500 micrograms/dose
- Respules® 2.5mg/mL
- Syrup 1.5mg/5mL
- Injection 500 micrograms/mL (2.5mg/5mL) Hospital only
Notes:
- Oral salbutamol: This is seldom needed but may be used in the management of asthma in adults. Use in children should be rare, for those unable to use any form of inhalation device, including paediatric spacer with facemask.
- If nebuliser solution is required Ventolin Nebules® offer cost savings (£9.24 per 28 days at 2.5mg QDS compared with £10.64 for Salamol Steri-neb®).
- Injections of beta agonists are only recommended for those patients unable to tolerate aminophylline infusion (use iv salbutamol) or for a small number of brittle asthmatics (use sc terbutaline).
Long Acting Beta2 Agonist - LABA
Formoterol fumerate
- Oxis Dry powder for inhalation, 6 micrograms per metered inhalation, 12 micrograms per metered inhalation
- Easyhaler Dry powder for inhalation, 12 micrograms per metered inhalation
- Atimos modulite Aerosal inhaltion, 12 micrograms/metered inhalation
- (Licensed 6 years and above)
Salmeterol
- Aerosol inhalation, 25micrograms per metered inhalation
Combined Long Acting Beta2 Agonist and Long Acting Muscarinic Antagonist - LABA + LAMA
Please follow COPD Inhaler Device Pathway in the appendix to help maintain device consistency and follow associated pathway in a step wise approach.
Duaklir® Genuair® (aclidinium / formoterol)
- 340mcg aclidinium/12mcg formoterol
- DPI - Dry powder inhaler
Spiolto Respimat® (tiotropium / olodaterol)
- 2.5mcg tiotropium/2.5mcg olodaterol
- Aerosol inhaler
Anoro Ellipta® (umeclidinium / vilanterol)
- 22mcg umeclidinium/55mcg vilanterol
- DPI
Ultibro Breezhaler® (indacaterol/glycopyronium)
- hard capsule (inhalation powder).
- 110mcg indacaterol/50mcg glcopyronium
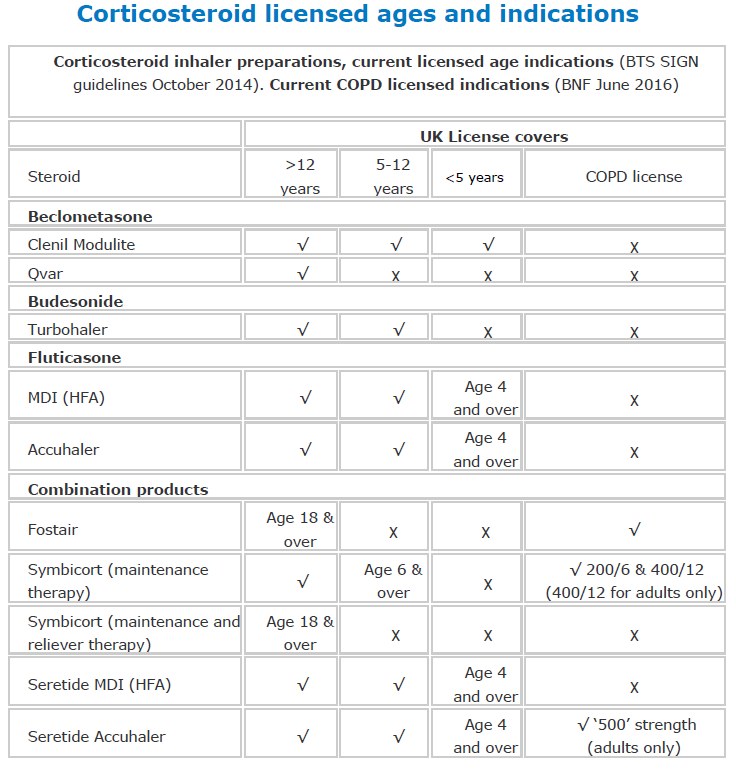
Corticosteroids
Airways disease, use of corticosteroids
Inhaled Corticosteroids - ICS
Important notes:
The lowest possible dose should be prescribed to maintain therapeutic effect.The total daily dose of inhaled corticosteroids should rarely be increased above:
Children: 800 micrograms beclometasone or budesonide; 400 micrograms fluticasone.
Adults: 2000 micrograms beclometasone or budesonide; 1000 micrograms fluticasone or Qvar.
Beclometasone dipropionate
- Inhaler MDI, Clenil Modulite® 50, 100, 200, 250 micrograms/dose
- Inhaler MDI, Qvar® 50, 100 micrograms/dose
Notes:
1. Clenil Modulite and Qvar are both CFC free metered dose inhalers (MDI). Clenil Modulite is licensed both for children and adults. Qvar is only licensed for children over 12.
2. The dose of Qvar will need adjusting when transferring a patient with well controlled asthma from other beclometasone inhalers. Initially a 100 microgram metered dose of Qvar should be prescribed for:
- 200 - 250 micrograms of beclometasone dipropionate or budesonide
- 100 micrograms of fluticasone propionate
Budesonide
- Dry powder for inhalation Pulmicort Turbohaler 100, 200, 400 micrograms/dose
- DPI Easyhaler 100, 200, 400 microgram/dose
- DPI Novolizer 200 micrograms/metered inhalation
- Respiratory solution 500 micrograms per 2mL, 1mg per2mL
Fluticasone
- Inhaler MDI 50, 125, 250 micrograms/dose
- Accuhaler 50, 100, 250 micrograms/dose
Inhaled Corticosteroids and Long Acting Beta2 Agonist - ICS + LABA
Fobumix Easyhaler® (Formoterol fumarate dihydrate/budesonide)
Breath-actuated dry powder inhaler (DPI):
- Fobumix Easyhaler 160/4.5 (Formoterol/budesonide); licensed for MART therapy
- Fobumix Easyhaler 320/9 (Formoterol/budesonide)
Fobumix Easyhaler 80/4.5 (Formoterol/budesonide); licensed for MART therapy
Fostair (beclometasone/formoterol)
MDI (metered dose inhaler)
- 100mcg beclometasone/6mcg formoterol; licensed for MART therapy
- 200mcg beclometasone/6mcg formoterol
NEXThaler DPI (dry powder inhaler)
- 100mcg beclometasone/6mcg formoterol; licensed for MART therapy
- 200mcg beclometasone/6mcg formoterol
Notes:
- MART = Maintenance And Reliever Therapy
Relvar Ellipta® (fluticasone furoate / vilanterol) TLS: Amber 2
- DPI, 92 mcg fluticasone furoate/22 mcg vilanterol (as trifenatate) - For Asthma and COPD
- DPI, 184 mcg fluticasone furoate/22 mcg vilanterol (as trifenatate) - For Asthma
Notes:
- Combined preparations reduce the flexibility of the individual components and are therefore not suitable for unstable asthmatics.
- The prescribing advisory group does NOT recommend the use of new products containing fluticasone for asthma. Our agreed strategy is to move away from fluticasone where possible in order to ensure that patients are stepped down and doses do not exceed those recommended by the MHRA.
Seretide® (fluticasone propionate / salmeterol)
- Accuhaler 100, 250, 500
- Evohaler MDI 50, 125, 250
Notes:
- The higher doses (Seretide Accuhaler 250 and 500, Evohaler 125 and 250) are only advised in step 4 of the management of asthma in adults.
- Seretide Accuhaler is licensed for COPD but the Evohaler is not - many patients with COPD are using the Seretide 250 Evohaler. The cost of Seretide Accuhaler 500 (one puff bd) is significantly less than Seretide 250 Evohaler (two puffs bd). It delivers exactly the same medication as for the same number of days but costs £20 less per unit.
- MHRA advice (Aug 2006): Prescribers of inhalers for asthma need to state clearly on the prescription which product should be dispensed by using the brand name (Clenil Modulite or Qvar) rather than prescribing the inhaler by using the generic name, beclometasone dipropionate CFC-free inhaler. If a pharmacist receives a generic prescription for a beclometasone dipropionate inhaler, they should establish which of the available branded products should be dispensed.
If switching to Qvar the step down dose may be less than half the current dose. The patient should be given an explicit statement to report to their doctor any worsening of their asthma. - MHRA Drug Safety Update (Sept 2010) Psychological and behavioural side effects may occur in association with use of inhaled and intranasal formulations of corticosteroids.
All patients (or their carers) should be informed of the important benefits of steroid treatment, and should be advised of these safety issues. All patients (or their carers) who receive steroids should receive a Patient Information Leaflet and be encouraged to read it. Patients should keep using their steroid medication, but should seek medical advice in the event of worrying symptoms or illness while taking steroids.
Symbicort (budesonide / formoterol)
- Turbohaler® dry powder inhaler 100/6; 200/6; 400/12
- MDI® 200/6
Trelegy Ellipta® (Vilanterol/Umeclidinium/Fluticasone)
- Inhaler DPI Vilanterol 22micrograms / Fluticasone 92 micrograms/ Umeclidinium 22 micrograms dose
Trimbow® (Beclometasone/Formoterol/Glycopyrronium)
-
MDI Inhaler 87mcg/5mcg/9mcg/dose
Notes:
- COPD patients with NO asthmatic features should be referred to AIRS / PCOC service before considering Triple Therapy Inhalers in line with the NEW COPD guidance.
- Some adult asthma patients may benefit from using Symbicort® as Maintenance And Reliever Therapy (SMART)
- Allowing patients to increase dose when their asthma worsens, avoiding the need for multiple inhalers. Only suitable for patients over 18 years where treatment with a combination of inhaled corticosteroid and LABA agonist is appropriate.
- Symbicort 400/12 is not licensed for use in this way. Fluticasone and salmeterol combination inhalers are not suitable for this regime.
- For all patients on high dose inhaled corticosteroids, review their treatment with an intention to step down treatment in view of the BTS guidelines. If this proves to be difficult, referral to a specialist is needed.
Guidance on inhaler devices and Nebulised therapy
|
|
Short acting beta2 agonists(SABA) |
Long acting beta2 agonists (LABA) |
Inhaled corticosteroids (ICS) |
CombinationLABA & ICS |
|
MDI (ideally used with spacer) |
Salbutamol |
Formoterol |
Clenil Modulite (Beclometasone), Fluticasone -(from step 3) |
Fostair (Beclometasone / Formoterol) OR Symbicort (budesonide / formoterol)- EITHER as 1st line combo Seretide 2nd only |
|
Easyhaler (DPI) |
Salbutamol |
Formoterol |
Budesonide |
Fobumix (Budesonide / Formoterol) |
|
Turbohaler (DPI) |
Terbutaline |
Formoterol |
Budesonide |
Symbicort |
|
Novoliser (DPI) |
Salbutamol |
No LABA available |
Budesonide |
No combo available |
|
Accuhaler (DPI) |
Salbutamol |
Salmeterol |
Fluticasone |
Seretide |
|
|
|
|
|
|
|
|
Short-acting Muscarinic Antagonist SAMA |
Long-acting Muscarinic Antagonist LAMA |
|
|
|
MDI (ideally used with spacer) |
Ipratropium bromide |
|
|
|
|
Handihaler (DPI) |
|
Tiotropium |
|
|
|
Respimat |
|
Tiotropium |
|
|
|
Genuair (DPI) |
|
Aclidinium? |
|
|
|
Breezhaler |
|
Glycopyrronium? |
|
|
MDI - Metered Dose Inhaler (Slow & steady breath)
DPI - Dry Powder Inhaler (Deep & forceful breath)
Respimat & Breezhaler - (Slow & steady breath)
Genuair (Deep & forceful breath)
Notes:
- Drug Safety Update. Pressurised metered dose inhalers (pMDI): risk of airway obstruction from aspiration of loose objects (July 2018)
Nebulised Therapy
General
- A nebuliser converts a solution of a drug into an aerosol for inhalation; In England and Wales nebulisers and compressors are not available on the NHS (but they are free of VAT).
- Before considering nebulised bronchodilators confirm optimal treatment with bronchodilators, good inhaler technique and consider other treatment options eg. in COPD, theophyllines and pulmonary rehabilitation.
- In adult asthma patients the requirement for regular nebulised bronchodilators would be an indication for referral.
Children
- Children requiring long-term nebulised therapy should be under the care of a consultant paediatrician.
- The provision of a home nebuliser for the management of acute asthmatic attacks is generally not recommended in children.
- The use of a nebuliser in acute asthmatic attacks in children under 2 years of age may result in a marked deterioration. Large volume spacers are preferable to nebulisers, where available. If it is felt appropriate to use a nebuliser in primary care, please be cautious.
Corticosteroids in children
BTS Sign guidelines state that specific written advice about steroid replacement in the event of a severe intercurrent illness should be part of the management plan for children treated with ≥800 micrograms per day of BDP or equivalent. Any child on this dose should be under the care of a specialist paediatrician for the duration of the treatment.
Mild croup is usually self-limiting, but treatment with a single dose of a corticosteroid (eg. dexamethasone 150 micrograms/kg) by mouth is of benefit.
Inhaled corticosteroids and adrenal suppression in children (Current Problems in Pharmacovigilance, vol. 28, Oct 2002).
Prescribers are reminded that the presenting symptoms of adrenal suppression and crisis are non-specific and include anorexia, abdominal pain, weight loss, tiredness, headache, nausea, and vomiting, decreased level of consciousness, hypoglycaemia and seizures. Situations that may potentially trigger acute adrenal crisis include infection, trauma, surgery or any rapid reduction in dosage.
NICE TA131: Inhaled corticosteroids for the treatment of chronic asthma in children under the age of 12 years (November 2007). The discontinuation of CFC-containing inhaler devices will affect the range of devices available but does not affect the guidance.
Oxygen
Oxygen prescribing for adults in the community
- Oxygen is a drug with benefits and adverse effects.
- Oxygen should be prescribed to ensure that it is safe and effective.
- Oxygen therapy is not effective in relieving breathlessness without hypoxia.
- GPs should refer patients to the oxygen assessment clinic and only prescribe oxygen in urgent palliative care situations where hypoxia has been proven (saturation
- Specialist nurses in the community and secondary care should normally complete the HOOF forms.
- HOOF forms are available from Oxygen suppliers
- Supplier details can be found in the BNF
- A patient's oxygen supplier will depend on their postcode
Types of home oxygen therapy (adapted from the Air Liquide clinicians guide)
Home Oxygen Therapy should only be prescribed for those patients that demonstrate hypoxia and can be ordered in the following formats:
- Long Term Oxygen Therapy (LTOT) - usually prescribed for periods of ≥15 hours per day with the aim of improving survival; Long Term Oxygen Therapy can prolong life and improve health status in selected patients with respiratory failure. LTOT is the only intervention, apart from smoking cessation to prolong survival in patients with COPD. Active smoking is not a contraindication for referral for LTOT, but patients should be counselled as to the risks.
- Ambulatory Oxygen Only - usually prescribed for patients who desaturate during exercise with the aim of improving exercise tolerance;
- LTOT with Ambulatory - for patients who meet both the above criteria;
- Short Burst Oxygen Therapy (SBOT). There is very little evidence indicating that Short Burst Oxygen Therapy is effective for relieving breathlessness and in many cases patients become psychologically dependant upon its use.
Who should be assessed for Home Oxygen Therapy?
Any of these features, in stable COPD, requires pulse oximetry:
- FEV1
- Cyanosis
- Polycythæmia
- Cor Pulmonale
If the SaO2 is less than 92% breathing air, the test should be repeated in a few weeks and if the SaO2remains
Indications:
Cluster Headache
- There is limited evidence of benefit, the recommended flow rate is 15 litres/minute, which is expensive and problematic for patients. This should only be initiated by a consultant neurologist.
Palliative care including heart failure
- There is limited evidence that in patients with persistent severe breathlessness in heart failure or terminal cancer those with hypoxia may benefit for SBOT, but no evidence of benefit that normoxaemic patients gain anything. SBOT is discouraged in terminal care unless they are both breathless and proven to be hypoxic.
Oxygen is safe and effective in acute asthma. Oxygen should be prescribed to achieve a target saturation of 94-98% for most acutely ill patients. For those at risk of CO2 retention, the oxygen saturation should be monitored by pulse oximetry, aiming for no more than 88-92%
Disadvantages of Oxygen:
Oxygen therapy can cause major toxicity to patients with COPD and other conditions through a range of mechanisms including CO2 retention.
There is an increase risk of house fires and the risks of fires need to be considered e.g. in smokers.
Home oxygen places an additional burden on families in terms of deliveries, accommodating the oxygen, use of open fires etc. Oxygen therapy is expensive and diverts resources away from those who need help.
BNF Oxygen Treatment Summary can be found here
In summary
Oxygen is a drug and should be prescribed after specialist assessment. GPs should not prescribe home oxygen except in urgent palliative care situations to relieve breathless when hypoxia is present. Oxygen therapy should not be given to patients without hypoxia.
Leukotriene receptor antagonists
Note: Neither sodium cromoglicate nor nedocromil are included in the current British Guidelines on the Management of Asthma. Compliance can be a problem because it needs to be given 3-4 times a day.
Montelukast
- Tablets 10mg (licensed >14 years)
- Chewable tablets 4mg (licensed 2-5 years)
- Chewable tablets 5mg (licensed 6-14 years)
Notes:
- Montelukast is a leukotriene receptor antagonist (LTRA) for use in the management of asthma (not COPD or any other condition). It is licensed as an adjunctive therapy in the control of mild to moderate and exercise induced asthma and can be used from the age of 6 months.
- Their possible role is as an add-on to, but not a substitute for, inhaled steroids. It is important to keep a small dose of inhaled corticosteroid.
- LTRAs are not effective in all patients. A response to treatment is usually very quick; however, there can be some patients that are late responders. A therapeutic trial should be given for 4 weeks. If after this time no response is observed, discontinue treatment.
- Montelukast can be used as an alternative at Step 2 of the British Thoracic Society asthma guidelines for children under five, or step 3 as an alternative to long acting beta2 agonist following no response for adults and children aged 5-12.
- Patients in whom LTRAs may be particularly effective are those with aspirin sensitivity, a large exercise induced component to the symptoms, highly atopic eczema and rhinitis.
Monoclonal antibodies
Omalizumab
- Prefilled Syringe 0.5mL (150mg/mL)
- Prefilled Syringe 1mL (150mg/mL)
Notes:
- Use as per NICE TA339: Omalizumab for previously treated chronic spontaneous urticaria (June 2015)
- Use as per NICE TA278: Omalizumab for treating severe persistent allergic asthma (April 2013)
Phosphodiesterase Type-4 Inhibitors
Roflumilast (Amber 3)
- Daxas® Tablets film-coated 500 micrograms
Notes:
- Use as per NICE TA461: Roflumilast for treating chronic obstructive pulmonary disease (July 2017)
Sympathomimetics (vasoconstrictor)
Ephedrine hydrochloride
- Injection 30mg in 1mL
Xanthines
Aminophylline
- Phyllocontin Continus® Modified release tablets 225mg
- Injection 250mg/10mL Min-I-Jet (Hospital only)
Theophylline
- Nuelin SA® Modified release tablets 175mg, 250mg
- Slo-Phyllin® Modified release capsules 60mg, 125mg, 250mg
- Uniphyllin Continus® Modified release tablets 200mg, 300mg, 400mg
Theophylline
Theophylline is seldom used, but may be used for severe asthma and COPD. Initiate therapy on a low dose and increase slowly if there is no therapeutic response.
Theophylline has a narrow margin between therapeutic and toxic dose and must be monitored closely with frequent blood tests. The therapeutic range for theophylline is 10-20mg/litre.
Monitoring:
- Initiation of IV therapy: 4-6 hours
- Subsequent levels for IV therapy: every 24 hours
- Initiation of oral therapy: 2-4 days
- Change of IV dose: 12-24 hours later
- Change of oral dose: 2-4 days
Interactions:
- The half-life is increased (giving higher theophylline levels) in heart failure, cirrhosis, and viral infections, in the elderly and by drugs such as cimetidine, ciprofloxacin, erythromycin, fluvoxamine, diltiazem, verapamil and oral contraceptives. The half-life is decreased (giving lower theophylline levels) in smokers, and in chronic alcoholism, and by drugs such as phenytoin, carbamazepine, rifampicin and barbiturates.
- Modified release preparations of theophylline should be prescribed by brand name. Prescribers should not interchange brands of theophylline due to the differences in bioavailability.
- Slo-phyllin® capsules can be opened and the granules sprinkled on soft food prior to administration.
- Vomiting may indicate a toxic dose.
Rescue Packs
Rescue Packs: Some patients with COPD are receiving multiple courses of antibiotics and/or prednisolone for exacerbations over a short space of time. In addition, patients are not being READ coded as receiving rescue medication.
Therefore, please note the following good practice points:-
Identifying suitable patients
1. Patients that may benefit from having rescue medication if they are able and willing to self-manage and have been given an action plan. Suitable patients will have a confirmed diagnosis of COPD and had 2 or more exacerbations in the past 12 months or has visited A&E/been admitted to hospital with an exacerbation of COPD.
Patient education
2. It is very important to ensure that patients understand when and how to use their antibiotics and steroids.
Audit
3. A READ code should be used to identify patients who have been issued rescue medication
4. A check should be made each time the patient requests additional items to make sure they are not over-using the medicines
5. When a patient asks for another supply of antibiotics and / or steroids they should be offered a review to check their current health status and ensure that the treatment was used appropriately. This may be a telephone consultation.
6. Rescue medications are not recommended on repeat prescription. If they are placed on repeat then there should be a maximum of 2 issues before reviewing patient.
Nebuliser solutions
Hypertonic Sodium Chloride (Prescribe by brand name)
MucoClear®
For acute bronchiolitis in infants
- Nebuliser solution, sodium chloride 3%
Resp-Ease®
- Nebuliser solution, sodium chloride 7%
Peak flow meters, inhaler devices and nebulisers
Peak flow meters
- Mini-Wright® Standard, EU range (60-800 litres/minute)
- Mini-Wright® Low range, EU range (30-400 litres/minute)
Notes:
- Mini-Wright® meters are recommended as they have an in-built non-return valve.
- Peak flow charts are a useful way of diagnosing and monitoring asthma. They should also be used in cases of suspected occupational asthma.
- All suspected cases of occupational asthma should be referred to the chest clinic for detailed peak flow monitoring.
Drug delivery devices
NICE Guidance for use of Inhaler devices within each age range can be found at:
NICE TA10: Inhaler systems (devices) in children under the age of 5 with chronic asthma, August 2000
NICE TA38: Inhaler devices for routine treatment of chronic asthma in older children (5-15 years), March 2002
Further guidance can be found here
Aerochamber Plus® Flow-Vu®
1. AeroChamber Plus® Flow-Vu® with small mask for infants (0-18 months) [Orange]
2. AeroChamber Plus® Flow-Vu® with medium mask for children (1-5 years) [Yellow]
3. AeroChamber Plus® Flow-Vu® with youth mouthpiece (5+ years) [Mint Green]
4. AeroChamber Plus® Flow-Vu® Adult mouthpiece [Blue]
5. AeroChamber Plus® Flow-Vu® adult with small mask [Purple]
6. AeroChamber Plus® Flow-Vu® adult with large mask [Blue]
Notes:
- One size mask doesn’t fit all – there are now multiple mask sizes to fit patients of different ages (e.g. a small and large adult mask, etc)
Patient Information Leaflets:
- How to use the Aerochamber Plus Flow VU Tidal Breathing
- How to use the Aerochamber Plus Flow VU
- How to clean the Aerochamber Plus Flow VU
- How to use the Aerochamber Plus Flow VU Children (small - medium mask)
- New Aerochamber Plus Flow VU, The full range
Volumatic® Spacer inhaler
- Paediatric with mask
Note:
- Wash spacers weekly, do NOT wipe dry.
- Replace every six to 12 months.
Guide to spacer devices prescribable on the NHS:
|
Spacer device |
Compatible MDI |
Masks available |
|
Volumatic |
Clenil Modulite, Ventolin, Flixotide, Serevent, Seretide |
Paediatric |
|
AeroChamber Plus® Flow-Vu® |
All pressurised metered dose inhalers |
Infant, child and Adult |
Nebuliser diluent
Sodium chloride
- Nebuliser solution 0.9% 2.5mL
Notes:
- Only to be initiated by a specialist for patients who need acute treatment or are unable to use inhaled devices effectively.
Traffic light status (TLS) explained:
- Green: Routine prescribing within licensed indication
- Amber 1: specialist recommendation followed by GP initiation and continuation
- Amber 2: specialist or GP initiation in line with local guideline after 1st line failure followed by GP continuation
- Amber 3: specialist initiation and stabilisation followed by GP continuation
- Amber SCG: specialist initiation and stabilisation followed by GP continuation in line with an agreed shared care guideline
- Red: Hospital or specialist prescribing only
Return to Chapter: 3. Respiratory System
Last updated by: Sheila Wood on 13-08-2019 10:45

%20v2.png)


