4.5 Pain
| First line drugs | Second line drugs | Specialist drugs | Secondary care drugs |
Traffic light status (TLS) explained:
- Green: Routine prescribing within licensed indication
- Amber 1: specialist recommendation followed by GP initiation and continuation
- Amber 2: specialist or GP initiation in line with local guideline after 1st line failure followed by GP continuation
- Amber 3: specialist initiation and stabilisation followed by GP continuation
- Amber SCG: specialist initiation and stabilisation followed by GP continuation in line with an agreed shared care guideline
- Red: Hospital or specialist prescribing only
Useful sources of information
Local guidelines:
- Clinical guideline: Chronic Pain Guideline (June 2016)
- Chronic Pain Guideline for Primary Care (June 2016)
Analgesics
Paracetamol
- Tablet 500mg
- Dispersible tablet 500mg
- Oral Suspension 120mg/5mL, 250mg/5mL,
- Suppository 125mg, 250mg, 500mg
- Intravenous infusion 500mg/50mL (anaesthetic team only)
- Intravenous infusion 1g/100mL (anaesthetic team only)
Notes:
- Should not be prescribed in Primary Care or at Discharge
- Advise patient to purchase, unless prescription is for long-term regular use
- Ensure IV paracetamol is prescribed by weight.
- Note the reduced dose for adults weighing less than 50kg.
- Paracetamol is usually the simple analgesia of choice.
- Paracetamol suppositories are very expensive and other forms should be substituted as soon as convenient.
- Avoid prescribing the 500mg in 5ml suspension as this is unlicensed and very expensive.
- Paracetamol is not licensed for use in children under 2 months old.
- Please see the current edition of the Children's BNF for further information
Nefopam hydrochloride (Amber 2)
- Tablet 30mg
Opioids
Buprenorphine CD
- Sublingual tablet 200 micrograms
- Patches (BuTrans®)(Amber 2) releasing 5 micrograms/hour for 7days, 10 micrograms/hour for 7days, 20 micrograms/hour for 7days
- Patches (Transtec®)(Amber 2) releasing 35 micrograms/hour for 96 hours, 52.5 micrograms/hour for 96 hours, 70 micrograms/hour for 96 hours
Notes:
- Buprenorphine patches are not suitable, or licensed, for use in the management of acute or intermittent pain
- Prescribers should ensure that patients and/or their careers are aware that Butrans® patches need to be applied at appropriate seven-day intervals to ensure that patients are not left in pain (because of too long an interval) and that the patches are not used wastefully (because of too short an interval).
- Remember to remove the old patch before application of new patch.
- To increase the dose, a higher strength patch should replace the patch that is currently being worn, rather than multiple patches being used
Co-codamol
- Tablets 8mg/500mg, 30mg/500mg
- Effervescent tablets 8mg/500mg, 30mg/500mg
Co-dydramol
- Tablet 10mg/500mg
Notes:
- Drug Safety Update: Co-dydramol: prescribe and dispense by strength to minimise risk of medication error (January 2018)
- Taking 8 tablets per day of soluble paracetamol or co-codamol will increase intake of sodium chloride by 8g daily. The Department of Health recommends a daily intake of sodium chloride of 6g. This may be a significant risk in patients with heart failure or hypertension. Dispersible preparations should be reserved only for patients who cannot swallow solid forms. They are also more expensive. Capsule preparations are of significant extra cost and are not included.
- Caplets are considered to be tablets and pharmacies will be reimbursed at the Drug Tariff price for paracetamol tablets.
Compound analgesics:
- There may be advantages to prescribing the opioid and non-opioid separately. This gives flexibility in both the adjustment of the doses and in the selection of the most appropriate combination.
- Compound preparations containing 8mg codeine phosphate may not provide significant additional relief of pain but is enough to cause opioid side effects and can complicate the treatment of over dosage.
- Please note: When co-codamol is prescribed and no strength stated, formulations containing codeine 8mg and paracetamol 500mg will be dispensed.
- Prescribers are reminded that tramadol/paracetamol combination preparations are not included in the formulary and should not be prescribed.
Codeine phosphate
- Tablet 15mg, 30mg
- Oral solution 25mg in 5mL
- Injection 60mg in 1mL CD (Hospital only)
Note:
- Codeine is effective for the relief of mild to moderate pain but is too constipating for long-term use.
- Codeine is contraindicated in all patients of any age known to be CYP2D6 ultra-rapid metabolisers
Diamorphine hydrochloride CD
- Injection 5mg, 10mg, 30mg, 100mg, 500mg
- Ayendi® Nasal Spray 720microgram/actuation
- Ayendi® Nasal Spray 1600microgram/actuation
Dihydrocodeine tartrate
- Tablets 30mg
Fentanyl CD (Amber 1)
- Patch 12 microgram/hour, for 3 days
- Patch 25 microgram/hour, for 3 days
- Patch 50 microgram/hour, for 3 days
- Patch 75 microgram/hour, for 3 days
- Patch 100 microgram/hour, for 3 day
Notes:
Drug Safety Update. Transdermal fentanyl patches: life-threatening and fatal opioid toxicity from accidental exposure, particularly in children (Oct 2018)
Effentora® (Fentanyl) CD
- Only for use by Willen Hospice
- Buccal tablets 100micrograms, 200micrograms, 400micrograms
|
Fentanyl, Immediate Release Commissioning Statement The statement below applies to prescribing outside NICE CG140 Opioids in Palliative Care. Prescribers should not initiate immediate release fentanyl for any new patient other than in line with NICE CG140 Opioids in Palliative Care. However, please note that immediate release fentanyl for palliative care is classified as RED (initiation and maintenance prescribing by specialist only) within MK, and even when use is in line with NICE CG140, primary care clinicians should not be asked to assume prescribing responsibilities. Immediate release fentanyl should be discontinued from primary care prescribing (deprescribed), with support from specialist services if necessary. Patient Information Leaflet can be found on the formulary website at https://www.formularymk.nhs.uk/includes/documents/Patient-information-Changes-to-immediate-release-fentanyl-prescribing.pdf
|
Meptazinol (Amber 1)
- Meptid® Tablet 200mg
- Meptid® Injection 100mg in 1mL (Hospital only)
Morphine sulfate CD
(Controlled Drug except for oral solution 10mg in 5mL)
4 hourly dosing preparations
- Oral solution 10mg in 5mL, 100mg in 5mL
- Tablet 10mg, 20mg, 50mg
- Injection 10mg in 1mL, 30mg in 1mL, 60mg in 2mL
12 hourly dosing preparations
- Zomorph® Modified Release capsule 10mg, 30mg, 60mg, 100mg, 200mg
- MST® Modified tablet 5mg
Infusion
- PCA (Accufusor) 100mg in 5mL
Notes:
- Morphine remains the most valuable opioid analgesic for severe pain although it frequently causes nausea and vomiting. In addition to pain relief, morphine also confers a state of euphoria and mental detachment.
- Zomorph® is the morphine sulphate MR preparation of choice because it is a capsule formulation that may be opened and the contents administered in semi solid food for patients with swallowing difficulties. Zomorph® is also licensed for use via gastric or gastronomy tubes (diameter >16F.G.). MST® lacks these advantages and is more expensive than Zomorph® in the community and Hospital and so its use is NOT recommended.
- Following titration, except for stat doses, for example for dressing changes, morphine should be given regularly every four hours (unless m/r preparations). If additional PRN doses are required the regular doasage should be reviewed and increased as appropriate.
- M/R preparations should be prescribed by brand name. ‘As required’ doses of immediate release product should be prescribed in case of breakthrough pain.
Oxycodone hydrochloride CD (Amber 1)
(specialist recommendation only and restricted use)
Oxycodone is restricted to use in:
- Palliative care patients intolerant of morphine
- Inpatient use for pain relief following THR/TKR protocol
- Chronic Pain consultant initiation according to criteria agreed in Chronic Pain Guideline
- Capsules 5mg, 10mg, 20mg
- Injection 10mg in 1mL, 50mg in 1mL
- Oral solution Sugar Free 5mg in 5mL
- Oral solution Sugar Free concentrate 10mg in 1mL
12 hourly dosing preparations
- Modified Release Tablets 5mg, 10mg, 15mg, 20mg, 30mg, 40mg, 60mg, 80mg, 120mg
Pethidine hydrochloride CD
- Tablet 50mg
- Injection 50mg in 1mL, 100mg in 2mL
Tapentadol CD (Amber SCG)
- MHRA Drug Safety Update (January 2019)https://www.gov.uk/drug-safety-update/tapentadol-palexia-risk-of-seizures-and-reports-of-serotonin-syndrome-when-co-administered-with-other-medicines
- Palexia SR® Modified Release Tablets 50mg, 100mg, 150mg, 200mg, 250mg
- For specialists initiation and presribing of first months' supply
Tramadol hydrochloride CD
- Capsules 50mg
- Injection 50mg in mL
Caution:
- Tramadol increases CNS serotonin levels and may cause increased serotogenic effects when given with antidepressants.
- The CSM have cautioned the use of Tramadol in patients with a history of epilepsy, as there may be an increased risk of convulsions.
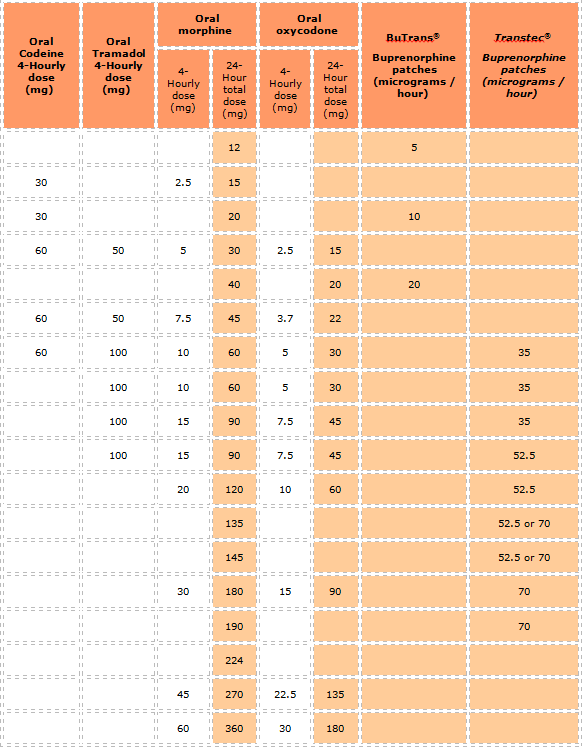
OPIOID APPROXIMATE EQUIVALENCE TABLE
Important Notes:
- The dosage equivalencies shown in the guide above are only approximate because comprehensive data are lacking and there is significant inter-individual variation. This variation is demonstrated, for example, with 100mg oral tramadol being quoted as approximately equivalent to between 10mg and 20mg of oral morphine
- These dosage equivalencies are based on data in palliative care medicine. No data could be found that would indicate different equivalencies for treating other patient groups
- When converting between opioids consider the total dosage per 24 hours being taken, including both regular and additional PRN doses. If the patient is on multiple opioids, it helps to convert them all to oral morphine equivalents
- When converting, particularly to a "stronger" opioid, consider an initial dose reduction of 25-50% from the calculated equianalgesic dosage (or choose a dosage at the lower end of the equivalent dosage range of the new opioid), and ensure that 4-hourly doses of PRN short-acting doses of opioid (eg Oramorph®) are prescribed for breakthrough pain
- However, a dosage reduction may not be appropriate if the original opioid taken at the prescribed dosage has failed to control the pain
- Once conversion has occurred, the dosage of the new opioid should be titrated carefully according to individual response and the patient monitored closely for the first 7-14 days for side effects and efficacy especially when switching at high doses.
Alfentanil CD
- Injection 500mcg per 1mL (2mL, 10mL amps)
- Injection 5mg per 1mL (1mL amps)
4.5.1 Migraine
National guidelines:
- NICE Clinical Guideline 150 Headaches in over 12s: diagnosis and management (Sept 2012, Last updated Nov 2015)
- NICE TA260 Botulinum toxin type A for the prevention of headaches in adults with chronic migraine (June 2012)
Local guideline:
Antimigraine drugs (see local guideline above)
Sumatriptan (first line choice triptan)
- Tablets 50mg, 100mg
- Injection 6mg/0.5mL syringe
Zolmitriptan
- Tablets 2.5mg
- Orodispersible tablets 2.5mg
Notes:
In general a triple drug combination taken together works best for the acute treatment of migraine:
- a 5HT1 Receptor agonists (known as a ‘Triptan’) e.g. Sumatriptan or Zolmitriptan
- a Non Steriodal Anti-inflammatory Drug (NSAID) e.g. Ibuprofen or Naproxen or Aspirin (this should be avoid in children under 16 years of age) or Paracetamol
- an Anti-Emetic eg. Metoclopramide, Prochlorperazine or Domperidone
Prophylaxis of Migraine (see local guideline above)
Propranolol
- Tablets 10mg, 40mg, 80mg, 160mg
- Modified release capsules 80mg, 160mg
Pizotifen
- Tablets 500 microgram, 1.5mg
Amitriptyline (off-label indication)
- Tablets 10mg, 25mg, 50mg
- Oral solution 50mg/5ml
Candesartan (off-label indication)
- Tablets 2mg, 4mg, 8mg, 16mg
Riboflavin (Vitamin B2) - Nutritional product
- Capsules 400mg
Note:
- Self-care product. Advise patients to buy over the counter
Topiramate (Amber 1)
- Tablets 25mg, 50mg, 100mg, 200mg
- Capsules 15mg, 25mg, 50mg (For patients with swallowing difficulties; Topamax® Sprinkle can be swallowed whole or sprinkled onto soft food and swallowed without chewing)
Notes:
- Contraindicated for migraine prophylaxis in pregnancy and in women of childbearing potential if not using a highly effective method of contraception.
- Useful agent for men and post-menopausal women in prophylaxis of migraine
- At doses of 200mg per day or greater, topiramate is a hepatic enzyme inducer and reduces the effectiveness of hormonal contraceptives
Duloxetine (off-label indication; Amber 1)
- Capsules 30mg, 60mg
Gabapentin (off-label indication; Amber 1) CD
- Capsules 100mg, 300mg 400mg
- Tablets 600mg, 800mg
- Oral solution 50mg/ml
Sodium Valproate (off-label indication; Amber 2)
- Tablets 200mg, 500mg
- Crushable tablets 100mg
- Oral solution 200mg/5ml
Notes:
- Valproate must no longer be used in any girls or women able to have children unless she agrees to comply with a pregnancy prevention programme.
- All women and girls who are prescribed valproate should contact their GP and arrange to have their treatment reviewed.
- Communication materials have been provided by the manufacturers to discuss the risk of abnormal pregnancy outcomes with women and girls of childbearing potential. See manufacturer’s “Risk Materials” on their SPC website
Botulinum toxin type A
- Use as per NICE TA260 Botulinum toxin type A for the prevention of headaches in adults with chronic migraine (June 2012)
4.5.2 Neuropathic pain
Notes:
- NICE Clinical Guideline CG173 - Neuropathic pain in adults: pharmacological management in non-specialist settings (November 2013)
Step 1
Amitriptyline hydrochloride
- Tablets 10mg,25mg,50mg
Imipramine
- Tablet 10mg, 25mg
Step 2
Gabapentin CD
- Capsule 100mg, 300mg, 400mg
Pregabalin CD
- Capsule 25mg, 50mg, 75mg, 100mg, 150mg, 200mg 225mg, 300mg
Notes:
1) Pregabalin is only to be used where amitriptyline and gabapentin have failed or are contraindicated.
2) Advice for prescribers on the risk of the misuse of pregabalin and gabapentin
Capsaicin
- Axsain® Cream 0.075% (Capsaicin 750 mcg per 1 gram)
Notes:
- For the management of local neuropathic pain in patients who cannot tolerate oral medication
- Apply sparingly 3–4 times daily (no more than every 4 hours)
- Review after 8 weeks
Step 3
Duloxetine (Amber 2)
- Capsule 30mg, 60mg
Lidocaine
Note: Classed as a "Low Value Medicine"
- Versatis® Plaster 5% 700mg/plaster
1) Licensed use: for the symptomatic relief of neuropathic pain associated with previous herpes zoster infection (post-herpetic neuralgia, PHN) in adults. For specialist pain clinic team initiation and stabilisation. Hospital to prescribe one months supply before asking GPs to continue to prescribe
2) Unlicensed use: for treatment of neuropathic pain in selected palliative patients with mesothelioma or chest wall disease. For specialist initiation and stabilisation. Hospital to prescribe one months supply before asking GPs to continue to prescribe
3) Unlicensed use: Patients with fractured rib. For specialist prescribing. Hospital to prescribe one months supply. GPs should not be asked to continue to prescribe.
|
Lidocaine Plasters Commissioning Statement
NOTE: This statement does not apply to patients who have been treated in line with NICE CG173 Neuropathic pain in adults: pharmacological management in non-`specialist settings but are still experiencing neuropathic pain associated with previous herpes zoster infection (post-herpetic neuralgia)
Apart from the exception above: Prescribers should not initiate lidocaine plasters for any new patient. Lidocaine plasters should be discontinued from primary care prescribing (deprescribed), with support from specialist services if necessary. If, in exceptional circumstances, there is a clinical need for lidocaine plasters to be prescribed in primary care, this should be undertaken in a cooperation agreement with a multi-disciplinary team and/or other healthcare professional. |
|
Patient Information Leaflet can be found on the formulary website at https://www.formularymk.nhs.uk/includes/documents/Patient-information-Changes-to-lidocaine-plaster-prescribing.pdf |
Capsaicin
- Qutenza® 179mg Cutaneous patch
Traffic light status (TLS) explained:
- Green: Routine prescribing within licensed indication
- Amber 1: specialist recommendation followed by GP initiation and continuation
- Amber 2: specialist or GP initiation in line with local guideline after 1st line failure followed by GP continuation
- Amber 3: specialist initiation and stabilisation followed by GP continuation
- Amber SCG: specialist initiation and stabilisation followed by GP continuation in line with an agreed shared care guideline
- Red: Hospital or specialist prescribing only
Return to Chapter: 4. Nervous System
Last updated by: Sheila Wood on 05-06-2019 12:24